Small molecules are more and more difficult to do, and macromolecules are developing strongly, which seems to be one of the important performances of drug R & D market in recent years. Many companies, boss and researchers have also started to focus their R & D on macromolecular biopharmaceuticals.
But objectively, the development mode of small molecule chemical drugs is obviously different from that of biomacromolecule drugs in many aspects. Rapid "cross-border" is really difficult for researchers. Therefore, the "polypeptide" drugs between small molecules and large molecules can be said to be a good transition turning point.
Of course, peptide drugs are not chicken ribs. They have been the focus of life scientists, chemists and pharmacists ever since they were found. At present, more than 80 drugs have been on the market, and some varieties have also developed into blockbusters with annual sales of billions of dollars. Therefore, whether it is the transformation of research and development direction or the transition of technology, the understanding of peptide drugs Digging deeply is one of the tasks that drug researchers must do!
Properties and characteristics of polypeptide drugs
Polypeptides, which can be said to exist widely in biological systems, are signal molecules, transport molecules and digestive molecules. As signal molecules, peptides control biological functions, such as cell division, mating, chemotaxis, pain, growth and immunity. Peptide synthesis has become one of the most powerful tools in biochemistry, pharmacology, immunology and biophysics;
As a transport molecule, polypeptide can promote the passage of ions through the cell membrane channel; as a digestive molecule, polypeptide plays an important role in the nutritional absorption of cells and complete organisms.
In addition, peptides can also be used as protective agents, such as antibiotics with excellent antibacterial and antiviral properties. Now, polypeptides are important commercial entities, which have been used in different treatment fields, such as diabetes, allergy, anti infection, obesity, diagnosis, tumor, arthritis and cardiovascular diseases.
Because peptide drugs mainly come from endogenous polypeptides or other natural polypeptides, in general, their structure is clear and their mechanism of action is clear. Many properties are often between small molecule chemicals and large molecule protein / antibody drugs. For example, compared with small molecule chemicals, the half-life is generally short, unstable, easy to be rapidly degraded in vivo, unstable preparations must be preserved at low temperature Low cost, high molecular medicine (especially long-chain peptide), etc;
Compared with macromolecular protein, it has better stability, less dosage, higher unit activity and lower cost, and the chemical synthesis technology of polypeptide is mature, easy to separate with impurities or by-products and high purity.
The above shortcomings, such as stability problems, some polypeptides can be modified or composed of stable compounds with other materials; cost problems, with the progress of science and technology, equipment update and process improvement, the cost can be better controlled.
The biggest problem of polypeptide drugs is that they can not be taken orally, mainly because they are easy to be degraded and difficult to pass through the intestinal mucosa. However, there are various alternative routes of drug delivery, such as subcutaneous injection, nasal spray, etc.
Classification of polypeptides
According to the source classification, polypeptides can be divided into bioactive polypeptides and synthetic polypeptides; according to the size classification, polypeptides can be divided into small peptide, medium peptide and large peptide. Small peptide refers to polypeptides with the number of amino acid residues less than 15, medium peptide refers to polypeptides with the number of amino acid residues between 15 and 50, and polypeptides with the number of amino acid residues greater than 50 are called large peptides;
According to the structure classification, polypeptides can be divided into homopolypeptides and heteropolypeptides, homopolypeptides contain straight chain peptide and circular peptide, heteropolypeptides contain pigment peptide, glycopeptide, lipopeptide and peptide; according to the function classification, polypeptides can be divided into exercise protein, hormone protein, toxin protein, peptide kinin, enzyme protein, peptide antibiotic and toxin peptide, etc.
Peptide R & D - representative events
In the early 20th century, Fischer synthesized peptides with specific sequences for the first time
In 1932, Bergmann et al. Used benzyloxycarbonyl to protect α - amino group, and peptide synthesis has developed to a certain extent.
In 1953, Vigneaud successfully synthesized the first peptide drug oxytocin
In 1963, Merrifield successfully synthesized peptide sequence with resin as solid-phase carrier, and established solid-phase peptide synthesis
In the late 1960s, Merrifield invented the first automatic peptide synthesizer and synthesized biological protease (124aa) for the first time
In the 1970s, the successive discovery of enkephalin and other opioid peptides in the brain made the research of neuropeptide enter a climax
In 1972, Lou carpino first used Fmoc to protect α - amino group, and it was quickly widely used
In 1984, Merrifield won the Nobel Prize in Chemistry for inventing solid-phase peptide synthesis technology
Research and development of peptide drugs
At present, more than 80 kinds of peptide drugs have been approved for marketing in the world, and the number of drugs is mainly distributed in tumor, diabetes, infection, immunity, cardiovascular, urinary and so on. At first, before the 1970s, less than one peptide drug entered the clinical trials every year;
In the 1970s, there was only about one entry into clinical trials each year; in the 1980s and 1990s, there were about five and 10 entry into clinical trials each year; in the first 10 years of the 21st century, the number of peptide drugs entering clinical trials increased, with an average of about 17 each year.
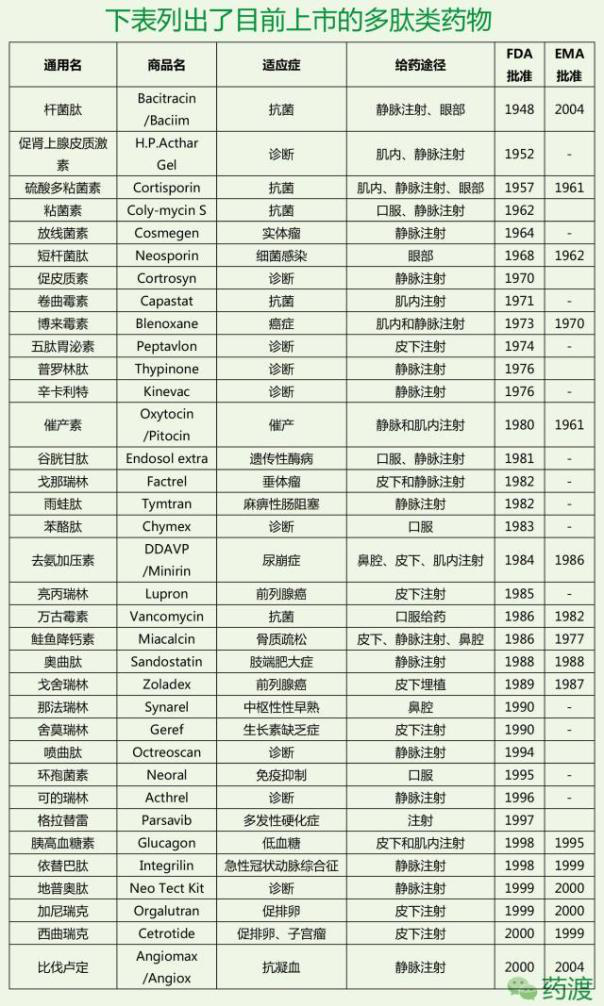
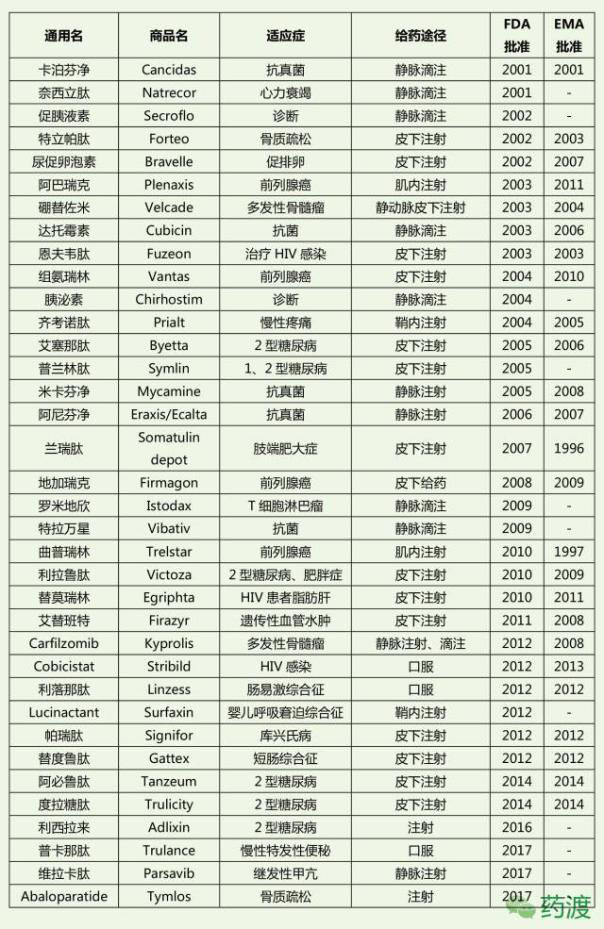
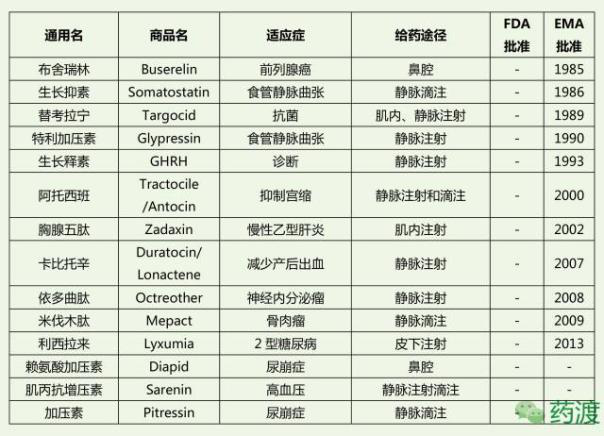
Peptide drug market
According to the transparency market research market report, the global peptide drug market scale in 2015 is nearly 20 billion US dollars, and will reach 23.7 billion US dollars in 2020, with a compound growth rate of about 2.8% from 2014 to 2020.
Peptide drugs are mainly used in the treatment of cancer, metabolic disorders related major diseases, which have a very important global market.
At present, there are more than 80 kinds of peptide drugs in the global drug market, 200-300 kinds of peptide drugs are in clinical trials, 500-600 kinds are in pre clinical trials, and more peptide drugs are in the laboratory research stage. It can be predicted that more and more peptide drugs will be approved by FDA to enter the drug market in the future. Some existing peptide drugs have a considerable market.
In terms of drug varieties, in 2016, glatirine acetate was still the top selling polypeptide drug, with sales of more than 4 billion US dollars for consecutive years, followed by lilalutide and growth hormone, with global sales of more than 2 billion US dollars, followed by octreotide, leuprorelin, tripatide, exenatide, goserelin, etc.
Methods for obtaining polypeptide drugs (the following are mainly technical aspects)
There are three main sources: natural extraction, biology and chemistry.
- Extraction of active components from natural products
There are active components in some natural products, which can be extracted from simple mixtures by solvent extraction. If the components of natural products are complex and the content of active components is very small, it is necessary to use enzymatic hydrolysis or microwave-assisted digestion to degrade the protein precursor. The active segments can be separated and detected by detection equipment, which can be quickly and effectively found in mixtures Active peptide.
Shengnuo bio PRODUCT
Best sales manager contact
| Contacts | Roleagh |
|---|---|
| Tel | 86-28-88203630 |
| Fax | 86-28-88203630 |
| roleagh@gmail.com | |
| 2539328606 | |
| LEI LI |
About Shengnuo
Chengdu Shengnuo Biotechnology Co., Ltd. has "Chengdu polypeptide drug engineering technology research center" in Chengdu, mainly engaged in polypeptide, polypeptide drug and beauty peptide research. Our zero defect has passed the FDA certification, and now it has become the first-class professional peptide drug and product development, technology transfer, technical service and peptide drug industry in the scale production and export of China's parks.